Migraine treatment with GON blockade at the level of C2: The tip of the iceberg and the submerged portion
Mustafa Karaoğlan
Department of Algology, Ordu State Hospital, Ordu, Turkey
Introduction
Migraine is a common and difficult-to-treat neurological disorder. One of the most important significant challenges in migraine treatment is the high discontinuation rates observed in preventive drugs. For this reason, preventive treatments involving peripheral nerve blocks have been widely used recently1. The most commonly used peripheral nerve block technique in the treatment of migraine is the blocking of the greater occipital nerve (GON)2. The effect of GON block is observable in the trigeminovascular system, which plays a vital role in the pathophysiology of migraine3-5.
Usually, GON blockade is performed blindly, based on anatomical landmarks at the superior nuchal line5. A study by Greher et al.6 described a more proximal block of the GON that was superficial in the obliquus capitis inferior muscle at the C2 level. Although several studies in the literature state that GON blockade at the C2 level is effective in occipital neuralgia and cervicogenic headache patients7-9, studies on GON blockade at the C2 level are mostly related to its use in the treatment of cervicogenic headache when the literature is reviewed. This form of block has not been adequately studied in the treatment of migraine, and the level of evidence in its current publications is not sufficient.
The aim of this mini-review is to discuss the role of C2-level GON blockade in the treatment of migraine, to examine its complications, and to question its future direction in light of the existing studies published so far in the literature.
Material and Methods
This mini-review of GON block for the treatment of migraine includes studies conducted with migraine patients aged >18 years who were treated with ultrasound-guided GON block at the C2 level. A search of Google Scholar and PubMed for the English language randomized controlled trials (RCTs), observational studies on GON block at the C2 level published between 2010 and 2023 was conducted using “greater occipital nerve”, “ultrasound-guided” and “migraine” as keywords. The last date of search was 1 March 2023.
All English-language article summaries that met the search criteria were reviewed. Figure 1 illustrates the article selection process and the number of articles at each step. Studies that focused on other primary headaches and those that added radiofrequency therapy to GON blockade therapy were excluded from this mini-review.
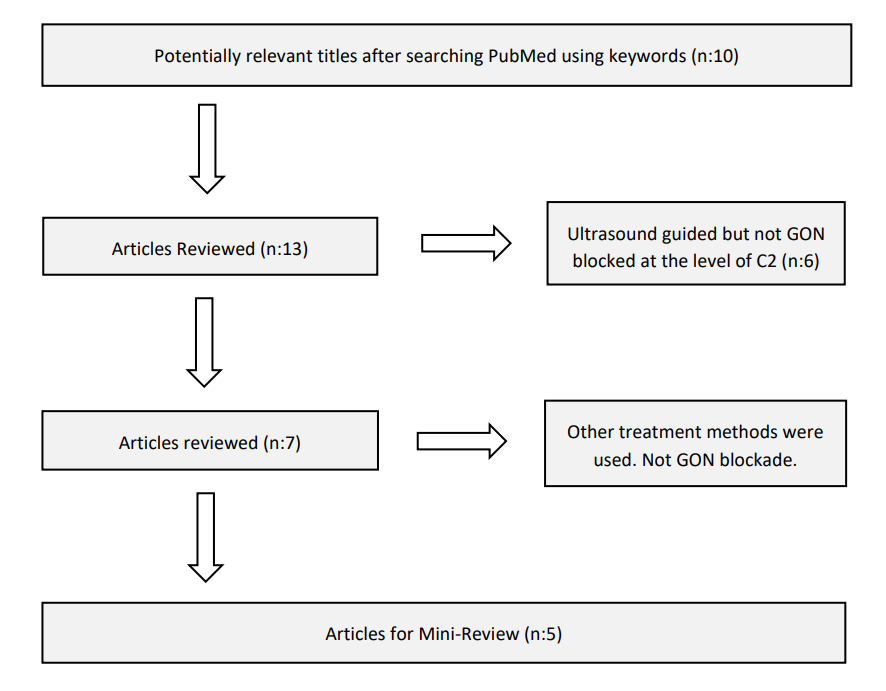
Figure 1: Flow diagram showing the progression of article selection and the number of articles at each step.
Results
In this mini-review, five trials involving GON blockade at the C2 level were summarized10-14. Although all patients had a diagnosis of migraine, one study also included medication overuse headache (MOH)14. In all of these studies, bupivacaine was preferred as the local anesthetic during GON blockade.
In the RCT conducted by Falmer et al.10 the effectiveness of distal GON blockade performed at the superior nuchal line level and GON blockade performed at the C2 level in chronic migraine patients was compared. In this RCT, 40 patients with a diagnosis of chronic migraine participated in the study and were divided into two groups. The distal GON blockade group was named Group D and included 20 patients, while the GON block at the C2 level group was named Group P and also included 20 patients. The blocks were performed as a single injection bilaterally or unilaterally based on the patient’s symptoms. During the blocking procedure, 1 ml of bupivacaine 0.5% was used for both levels and an additional 1 ml of 40 mg methylprednisolone was added. Analysis of NRS pain scores at 1-month post-procedure (primary endpoint for the study) revealed a significant reduction from baseline in the proximal group. However, this result was not significant in the distal GON blockade group (mean difference in Group P: -1.85 [95%CI -3.29 to -0.42); p=0.014].
Similarly, NRS pain scores decreased significantly in both groups at 24 hours after the procedure and at week 1 compared to baseline, and in the proximal group at 3 months follow-up. When compared between groups, both the NRS for sleep division and the average hours of restful sleep hours per night were similar during each follow-up period. However, within-group analysis showed an improvement in NRS for sleep interruption compared to baseline at week 1 in both groups. Compared to baseline, the number of headache days per week was significantly reduced at month 1 in the proximal injection group, but there was no significant difference between the two groups. Patient satisfaction was similar for both groups and almost 60% of patients were satisfied with the result.
In terms of procedural outcomes, the time required to perform the block was similar for both the proximal and distal approaches. The mean NRS for procedural discomfort (0 indicative of no discomfort and 10 indicative of serious discomfort) showed mild to moderate discomfort and was similar in both injection groups (4.50 vs. 3.39, p=0.240).
In the study conducted by Balta11, a total of 25 chronic migraine patients were included as a single group and followed up for 6 months. All of the blocks were made at the C2 level and 1 ml of bupivacaine 0.5% was used for the blockades. GON blockades were repeated weekly and applied a total of 4 times in the study.
According to the study, clinical success was defined as a 30% reduction in headache days in one month. The reductions in 1st, 3rd, and 6th months post-blocking were 84% (n = 21), 72% (n = 18), and 68% (n = 17), respectively. The study found no statistically significant relationship between clinical success after the 6-month visit and age, disease duration, initial attack duration, pain severity, MIDAS grades, HIT-6 levels, BDI scores and BAI scores (p=0.279, 0.193, 0.160, 0.826, 0.068, 0.207, 0.389, and 0.076 respectively). Additionally, there was no statistically significant difference between bilateral and unilateral migraine headache symptoms in terms of clinical success at the 6-month visit (p = 0.389, χ2 = 1.634).
A total of 63 patients were included in the retrospective study conducted by Karaoglan et al.,12 which examined the clinical results of distal and C2 blockade. During this study, patients were divided into two groups; the distal group was named DOGON, and 31 patients were included while the second group, C2GON, included 32 patients who received GON blockade at the C2 level. In the DOGON group, 1.5 ml of bupivacaine 0.5% was administered bilaterally with anatomical markers without the use of ultrasound. In the C2GON group, a 4 ml bupivacaine 0.5% block was applied bilaterally. All blocks were repeated weekly, and a total of 4 blocks were applied over a 1-month period.
When the findings were evaluated together, it was observed that the number of migraine attacks and mild attacks (VAS>4) in the 30 days, which differed significantly before the treatment, did not show a significant difference in the 1st month, but showed an increase in the difference again in the 3rd month.
The groups that did not show a significant difference in terms of the number of days of severe headache and the mean duration of headache (hours) 30 days before the treatment, showed a significant difference in the 1st month, but this significant difference closed in the 3rd month.
Triptan use, which showed a significant difference before treatment, did not show a significant difference in the 1st and 3rd months. Additionally, the number of severe attacks in 30 days, total analgesic use, and the number of days with headache did not differ significantly before and after treatment (p > 0.05).
In addition, when the complications observed between both groups, there were complications in 25% of the C2GON group and 12.9% of the DOGON group. Dizziness and cerebellar like syndrome were found in the C2GON group, while postprocedural pain was observed in the DOGON group. However, it was observed that all complications were temporary. As a result, there was no significant difference between the groups in terms of complications (p > 0.05).
In another study conducted by Karaoglan et al.,12 bilateral and unilateral GON blockade at the C2 level was administered. The study included 52 patients with chronic migraine, 25 of whom received bilateral blockade and 27 received unilateral blockade. Both groups were administered 4 ml of bupivacaine 0.5% and the block was repeated once a week for a total of 4 blocks. No significant difference was observed between the two groups in all data. However, a detailed examination of complications revealed that no complications were observed after unilateral application, while complications were observed in 20% of those who received bilateral application (Table 1).
In another study by Karaoglan, Onabotulinum toxin A (onA) and GON blockade at the C2 level were compared. In this study, GON blockade at the C2 level and onA therapy were administered concurrently. A total of 85 patients with chronic migraine were included in the study, 48 of whom also had a diagnosis of MOH. The local anesthetic used for all GON blockades administered bilaterally was 0.125%. A total of 4 ml injections were given, and injections were repeated once a week for a total of 4 injections. All onA treatment for migraine was administered as a single session. No significant difference was observed between the groups in terms of the application of GON blockade alone and its application with onA. Moreover, these two treatment modalities were found to be superior to the group in which onA treatment was the only treatment option (Table 1).
Table 1: Characteristics of studies (Abbreviations:- BoNT-A: Onabotulinum Toxin A treatment for chronic migraine; C2: Cervical 2 vertebra body; C2GON: GON block at the C2 level; GDOGON: Distal occipital GON block; GONB: GON block at the C2 level, GoNT-A: Dual treatment of GON block at C2 level and onabotulinum toxin A treatment.)
|
First author; year |
Number of patients; study design |
Side effects |
Headache types |
Drugs |
Number of blocks; Block locations |
Results |
|
Flamer et al. |
Included:40; randomized, double-blinded Group P: GON block at the level of C2 with local anesthetics and corticosteroid (n:20) Group D: GON block at distal level with local anesthetics and corticosteroid (n:20) |
No serious adverse effects |
Chronic migraine |
1 ml bupivacaine (0,5%) + 1 ml |
Based on symptoms; bilateral or only one block was performed.
Facial plane block at the C2 level or at the level of superior nuchal line 2-3 cm lateral to the external occipital protuberance. |
A mixed between-subjects and within-subjects ANOVA was conducted to assess the impact of the two interventions (Distal GON and Proximal GON blocks) on the NRS pain scores, across five time points (preprocedural, and 24 hours, 1 week, 1 month, and at 3 months after the procedure).
There was no significant interaction between the two groups and time (p=0.809). |
|
Balta 202111 |
Included: 25 |
Dizziness (n:4) |
Chronic migraine |
1,5 ml of bupivacaine 0,5% |
Bilateral application; 4 weekly injections; Facial plane at the level of C2 block |
A statistically significant difference in the frequency of headache attacks, number of headache days, duration of headache attacks, and values of pain intensity was found between the baseline and control visits (p < 0.001). Further, a statistically the baseline evaluation of chronic migraine with medication overuse was 84% (n = 21); it regressed in 24% (n = 6) at the 6-month visit. |
|
Karaoglan et al. |
Included; 63; retrospective two group; DOGON: n: 31, C2GON: n: 32 |
C2GON: n:8 (Cerebellar like syndrome n:4; dizziness n:4) |
Episodic Migraine |
4 ml of bupivacaine 0,5% |
Bilateral application; 4 weekly injections for two groups Facial plane block at the C2 level or at the level of superior nuchal line 2-3 cm lateral to the external occipital protuberance |
The groups that did not show a significant difference in terms of the number of days of severe headache and the mean duration of headache (hours) at 30 days before the treatment showed a significant difference in the 1st month, but this significant difference closed in the 3rd month. Triptan use, which showed a significant difference before treatment, did not differ significantly at 1 and 3 months. In addition, the number of severe attacks in 30 days, total analgesic use, and the number of days with headache did not differ significantly before and after treatment (p > 0.05). |
|
Karaoglan et al. |
Included 52; retrospective Unilateral n:27 Bilateral; n:25 |
No complication in unilateral group; vertigo n:1, dizziness n:3 ; Cerebellar like syndrome n:1 |
Chronic migraine |
4 ml of bupivacaine 0,5% |
Bilateral or unilateral application 4 weekly injection for two group Facial plane block at the C2 level |
In both groups, the number of days with headache in 30 days, the average duration of headache (h), the highest VAS score in 30 days, and total analgesic use in 30 days decreased in the 1st month compared to the pre-treatment period and increased in 3 months.
However, results of both the 1st and 3rd months were significantly lower than before treatment (p < 0.05). Although the positive effect, which was greater in the 1st month, decreased partially in the 3rd month, it was still significant compared to the pre-treatment, and this finding showed that the clinical effect continued until the 3rd month in both groups. |
|
Karaoglan et al. |
Included 85; retrospective; BoNT-A: 27, GONB: 30, GoNT-A: 28 |
Only ptosis for BoNT-A g Difficult in concentrating n:4; local site bleeding n:2; non-specific occipital headache n:2, unilateral ataxia n:2 for GONB group Difficult in concentrating n: 3; dizziness n:3, local site bleeding n:1, non-specific occipital headache n:1; for GoNT-A group. |
Chronic migraine, |
4 ml of bupivacaine 0,125% |
Bilateral application Facial plane block at the C2 level |
When VAS scores were evaluated statistically, both GONB and GoNT-A applications showed a statistically significant more reduction than BoNT-A application (p < 0.05).
The decrease in the VAS score of GONB and GoNT-A applications did not show a statistical difference (p > 0.05). |
Discussion
Until now, the efficacy of GON blockades at the C2 level in the treatment of migraine has been demonstrated in a total of 5 studies, only one of which was an RCT. However, the level of evidence provided by these studies is still insufficient.
Blocking Techniques
Although various block techniques have been defined for GON blockade performed at the distal level, the technique used for the C2 level was consistent across all the studies. It would be risky to employ different strategies for the technique due to the use of ultrasound, the proximity of the vertebral artery to the block site, and the planar block. Therefore, in the five studies we examined, the GON blockade technique used for the C2 level was similar. For now, only one technique appears to have been adopted from a technical standpoint.
Drugs and Doses
The effect of local anesthetic agents is associated with the reversible blockade of sodium channels within the nerve fibers. They act on demyelinated C-fibers and myelinated A-delta fibers, which prevents the transmission of pain signals by disrupting the depolarization of nerve15. Bupivacaine was the most commonly used agent for GON blockade at the level of C2.
In all the studies we reviewed in the mini-review, bupivacaine was the preferred local anesthetic. In the studies by Flamer et al.10 and Balta11, patients were administered bupivacaine 0.5% as a total dose of only 1 ml. Flamer10 added 1 ml of 40 mg methylprednisolone, increasing the total dose to 2 ml. We observed that corticosteroid use, which is still controversial in the distal GON blockade for the treatment of migraine, is also used in the C2 level GON blockade. As the corticosteroid used here was used in only one study and in each group, we currently do not have data on the benefit of corticosteroid use in GON blockade at the C2 level.
In all of the studies conducted by Karaoglan et al.,12-14 the total solution dose of 4 ml was applied. However, there were differences in the doses used among these three studies. Notably, in Karaoglan’s last study14, although the total bupivacaine dose was kept constant at 4 ml, bupivacaine was diluted to 0.125 %.
Single or Recurrent Injections
In the RCT performed by Flamer et al. GON blockade was administered once to patients with migraine headaches, and if the pain was unilateral, the blockade was applied only on that side. In cases of bilateral pain, GON blockade was applied bilaterally. Although the pain was defined as unilateral in this study, migraine can cause bilateral pain in progressive attacks due to its nature. The most controversial aspect of this study was the injection method. In other studies, GON blockade was repeated weekly and a total of 4 injections were given. Currently, there is no study that demonstrates the difference between a single injection and repeated injection.
Complications and Side-effects
When we reviewed all studies, no serious side effects were reported in studies that used 1 ml of local anesthetic10,11. In a study by Balta11 with 25 patients, dizziness was observed in 4 patients. Although this side effect was also observed after distal blockade, its incidence was not that high1.
Serious side effects were not observed in GON blockade administered at the C2 level with a total dose of 4 ml in studies12-14. However, in the study by Karaoglan et al.12 in which undiluted bupivacaine was bilaterally administered a side effect defined as “cerebellar like syndrome” by the author and not previously reported in the literature, was observed and supported by video. While explaining this, the author stated that they predicted that the GON at the C2 level might have a connection with the cerebellar region, but there was still insufficient evidence to support this information. Although the author tried to explain this complication in this way, the reason why this complication was less common in unilateral blocks remained a mystery. All of these symptoms resolved within 6 hours with no permanent adverse effects. Although this side effect was not serious, it could have had dramatic consequences for the patient, and therefore, in future studies, the author used diluted local for GON blockade at the C2 level.
Submerged Portion of C2 GON Blockade
Since there was insufficient evidence in the literature, the information in this section is based on our clinical experience and predictions. Unfortunately, we cannot provide more than an interpretation. One of the biggest advantages of GON block at the C2 level, as a fascial block, is that it offers more effective blocking by using more local anesthetics. However, in our studies, we observed that the use of 4 ml of bupivacaine 0.5% significantly increased the occurrence of side effects. Therefore, we diluted the bupivacaine dose while keeping the advantage of 4 ml. We observed fewer side effects with bupivacaine 0.125%. Even at the diluted dose of bupivacaine, significant dizziness and difficulty concentrating were more common than with distal GON blockade, and patients reported it as a bothersome side effect. It is possible to predict that these symptoms could negatively affect the continuation of treatment.
In our clinical practice, we observed a patient experiencing severe and radicular pain in the shoulder and arm after lidocaine was used instead of bupivacaine during C2 GON blockade. As these symptoms persisted for about a week and had a dramatic effect on the patient, we have temporarily discontinued the use of lidocaine in our clinical practice. Although this symptom occurred within the 30-minutes after GON blockade; it was not possible to definitively attribute it to the blockade. In our examinations, we could not detect any other pathology that could explain the symptoms. However, the symptoms were completely resolved after a week. We have not encountered such a symptom in any of the patients in whom we performed the C2 GON blockade using bupivacaine.
During the GON block performed at the C2 level, the injection site was reported by patients to be uncomfortable and painful, which was a significant concern for us. Using 1 ml of bupivacaine was not deemed a practical option as the same dose could be used for distal GON blockade. We suggest prioritizing the use of distal GON blockade in the treatment of migraine, as there are numerous studies supporting its safety, and the bothersome side effects of C2 GON blockade with 4 ml of solution, although not serious, make it a less desirable option.
The main future use of GON blockade at the C2 level seemed to be directed towards other non-migraine primary headaches. Potential targets for GON blockade at the C2 level included postural puncture headache, cervicogenic headache, occipital headache, and cluster headache syndromes.
The additional benefit of adding corticosteroids to GON blockade for cluster headache has been reported2. However, adding steroids to the distal GON blockade could lead to undesirable side effects, including the most feared side effect among patients, which is local alopecia. It was not possible to experience this side effect during the block at the C2 level due to the relevant anatomy. In conclusion, while C2 GON blockade has demonstrated some superiority over distal GON blockade for the treatment of migraine, we do not anticipate it having a significant impact on clinical practice. However, the fact that this blockade can cause cerebellar side effects in migraine patients may support further examination of the role of residual cerebellum in migraine pathophysiology, potentially leading to new target regions for pharmacological interventions16-18. This potential avenue of research could open up new horizons for treating migraine and could be seen as the most important benefit of C2 GON blockade for migraine patients.
References
- Inan LE, Inan N, Unal-Artik HA, et al. Greater occipital nerve block in migraine prophylaxis: Narrative review. Cephalalgia: an international journal of headache. 2019; 39(7): 908-920. https://doi.org/10.1177/0333102418821669
- Stern JI, Chiang CC, Kissoon NR, et al. Narrative review of peripheral nerve blocks for the management of headache. Headache. 2022; 62(9): 1077-1092. https://doi.org/10.1111/head.14385
- Piovesan EJ, Kowacs PA, Tatsui CE, et al. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia: an international journal of headache. 2001; 21(2): 107-109. https://doi.org/10.1046/j.1468-2982.2001.00166.x
- Selekler MH. [Greater occipital nerve blockade: trigeminicervical system and clinical applications in primary headaches]. The journal of the Turkish Society of Algology. 2008; 20(3): 6-13.
- Chowdhury D, Datta D, Mundra A. Role of Greater Occipital Nerve Block in Headache Disorders: A Narrative Review. Neurology India. 2021; 69(Supplement): S228-S256. https://doi.org/10.4103/0028-3886.315993
- Greher M, Moriggl B, Curatolo M, et al. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. British journal of anaesthesia. 2010; 104(5): 637-642. https://doi.org/10.1093/bja/aeq052
- Pingree MJ, Sole JS, O' Brien TG, et al. Clinical Efficacy of an Ultrasound-Guided Greater Occipital Nerve Block at the Level of C2. Regional anesthesia and pain medicine. 2017; 42(1): 99-104. https://doi.org/10.1097/AAP.0000000000000513
- Zipfel J, Kastler A, Tatu L, et al. Ultrasound-Guided Intermediate Site Greater Occipital Nerve Infiltration: A Technical Feasibility Study. Pain physician. 2016; 19(7): E1027-E1034.
- Lauretti GR, Correa SW, Mattos AL. Efficacy of the Greater Occipital Nerve Block for Cervicogenic Headache: Comparing Classical and Subcompartmental Techniques. Pain practice: the official journal of World Institute of Pain. 2015; 15(7): 654-661. https://doi.org/10.1111/papr.12228
- Flamer D, Alakkad H, Soneji N, et al. Comparison of two ultrasound-guided techniques for greater occipital nerve injections in chronic migraine: a double-blind, randomized, controlled trial. Regional anesthesia and pain medicine. 2019; 44(5): 595-603. https://doi.org/10.1136/rapm-2018-100306
- Balta S. Midterm clinical outcomes of ultrasound-guided bilateral C2 level greater occipital nerve block in patients with chronic migraine. Neurology Asia. 2021.
- Karaoglan M, Inan LE. A comparison of the clinical efficacy of GON block at the C2 level and GON block at the classical distal occipital level in the treatment of migraine. Clinical neurology and neurosurgery. 2022; 215: 107190. https://doi.org/10.1016/j.clineuro.2022.107190
- Karaoglan M, Durmus IE, Kucukcay B, et al. Comparison of the clinical efficacy of bilateral and unilateral GON blockade at the C2 level in chronic migraine. Neurological Sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022; 43(5): 3297-3303. https://doi.org/10.1007/s10072-021-05739-5
- Karaoglan M. Three men in a boat: The comparison of the combination therapy of botulinum toxin and greater occipital nerve block with bupivacaine, with botulinum toxin monotherapy in the management of chronic migraine. Clinical neurology and neurosurgery. 2023; 226: 107609. https://doi.org/10.1016/j.clineuro.2023.107609
- Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiology clinics of North America. 2000; 18(2): 217-33. https://doi.org/10.1016/s0889-8537(05)70161-9
- Noseda R. Cerebro-Cerebellar Networks in Migraine Symptoms and Headache. Frontiers in pain research (Lausanne, Switzerland). 2022; 3: 940923. https://doi.org/10.3389/fpain.2022.940923
- Vincent M, Hadjikhani N. The cerebellum and migraine. Headache. 2007; 47(6): 820-33. https://doi.org/10.1111/j.1526-4610.2006.00715.x
- Wang M, Tutt JO, Dorricott NO, et al. Involvement of the cerebellum in migraine. Frontiers in systems neuroscience. 2022; 16: 984406. https://doi.org/10.3389/fnsys.2022.984406