The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behcet's Disease: A Systematic Review
Gerald E. Davis II1,2, George Sarandev1, Alexander T. Vaughan1, Kamal Al-Eryani3, Reyes Enciso4*
1Advanced graduate, Master of Science Program in Orofacial Pain and Oral Medicine, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA
2Assistant Dean of Academic Affairs, Assistant Professor, Restorative Dentistry, Meharry Medical College, School of Dentistry, Nashville, Tennessee, USA
3Assistant Professor of Clinical Dentistry, Division of Periodontology, Dental Hygiene & Diagnostic Sciences, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA
4Associate Professor (Instructional), Division of Dental Public Health and Pediatric Dentistry, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA
Abstract
Background: Current treatments for pemphigus and Behcet's disease, such as corticosteroids, have long-term serious adverse effects.
Objective: The objective of this systematic review was to evaluate the efficacy of biologic agents (biopharmaceuticals manufactured via a biological source) on the treatment of intraoral lesions associated with pemphigus and Behcet's disease compared to glucocorticoids or placebo.
Methods: PubMed, Web of Science, Cochrane Library, and EMBASE were searched for randomized controlled studies up to January 2019. Bias was assessed with the risk of bias tool.
Results: Out of 740 references retrieved, only four randomized controlled trials (RCTs) were included, comprised of a total of 158 subjects (138 pemphigus and 20 Behcet's disease). All studies were assessed at high risk of bias. Heterogeneity of data prevented the authors from performing a meta-analysis. Infliximab or rituximab with short-term prednisone showed higher safety and lowered cumulative prednisone dose than prednisone alone in the treatment of pemphigus. Subcutaneous injection of etanercept provided 45% of patients free of ulcers compared to 5% in the placebo group in one study with Behcet's disease; however, no difference was found in pemphigus patients.
Conclusion: Though biological agents alone or in combination with prednisone showed favorable results in three RCTs compared to prednisone alone or placebo, a meta-analysis could not be undertaken due to high heterogeneity. Results are inconclusive, and larger, well-designed RCTs are needed.
Introduction
Pemphigus, Behcet's disease, mucous membrane pemphigoid, oral lichen planus (OLP), and recurrent aphthous ulcers are immune-mediated disorders, which commonly have oral manifestations. Though there are variations in the intraoral presentation of these conditions, they often present with ulcerations1.
Pemphigus is an autoimmune condition with three major variants: Pemphigus Vulgaris (PV), Pemphigus Foliaceous (PF), and Paraneoplastic Pemphigus (PNP)2. Only PV and PNP have common oral involvement3. In PV, IgG antibodies targeted against desmoglein-3 (Dsg-3), a protein responsible for cell-cell adhesion, lead to loss of adhesion (acantholysis) and blister formation4. In addition to Dsg-3, patients with PNP also have antibodies against Dsg-1 and plakin family3. Moreover, PNP is usually associated with underlying malignancy, such as non-Hodgkin lymphoma3. Although PV and PNP share many clinical findings with other vesiculobullous lesions, it is still very possible to differentiate between them using their unique clinical and histopathological features5.
Behcet's disease is an autoimmune disease that targets small blood vessels, causing vasculitis. This leads to clinical effects such as uveitis and oral and genital ulcerations6. Currently, there is no cure for Behcet's disease; however, it has been managed using immunosuppressive therapy. The cause of Behcet's disease is unknown, but there are genetic predispositions for the disease, namely, in the Middle East along the path of the original Silk Road7. HLA-B51 proteins help aid in the precipitation of Behcet's disease due to chemotaxis hyper-function7.
Mucous Membrane Pemphigoid (MMP) is an autoimmune condition that targets hemidesmosomes; lichen planus is a chronic condition with both extraoral and intraoral effects.
Recurrent Aphthous Stomatitis (RAS) are non-specific ulcers of unknown etiology and may be present secondary to systemic diseases8. Thalidomide is considered to be an effective therapy to treat RAS, and it can be used in refractory cases with complete remission in 85-90 % of patients9.
In addition to palliative therapy such as topical anesthetics, primary therapy for these immune-mediated intraoral lesions consists of glucocorticosteroids, either given topically, as is the case in MMP, Behcet's disease, and OLP or systemically, as in pemphigus for example10-13. When lesions in MMP and OLP are recalcitrant to topical glucocorticoids, systemic glucocorticoids are often attempted12. However, long-term management with systemic glucocorticoids may present with minor side effects (such as acne) to major (glaucoma, cataract, adrenal atrophy, Cushing’s syndrome, increased glucose levels, or cerebral atrophy) outcomes14. Although steroids significantly reduced the mortality associated with pemphigus in one retrospective multi-center cohort study, serious side effects are a common cause of morbidity and mortality15. It has recently been proposed that treating pemphigus with a combination of rituximab and short-term prednisone is safer and more effective than high doses of corticosteroids16. The use of biologic agents has emerged as an additional line of treatment for ineffective immunosuppressive therapy with corticosteroids. Infliximab and rituximab are chimeric monoclonal antibodies, while etanercept is a chimeric fusion protein. Infliximab is a drug that was developed in 1993, and it functions by acting as a TNF-α antagonist17. Rituximab conversely targets CD-2018. Etanercept also inhibits TNF-α19.
The objective of this systematic review was to evaluate the efficacy of biologic agents (biopharmaceuticals manufactured via a biological source) on the treatment of intraoral lesions associated with pemphigus and Behcet's disease compared to glucocorticoids or placebo.
Materials and Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement20 and was registered with PROSPERO #CRD42019128312.
The PICOS (Patient, Intervention, Comparison, Outcome, Setting) question was:
P: Adult patients with oral lesions associated with pemphigus, MMP, OLP, recurrent aphthous ulcers and Behcetss;
I: Biologic agents alone or in combination with corticosteroid;
C: Placebo or corticosteroid;
O: Reduction in number of oral lesions, number of responders, any quality of life index, duration to the healing of existing blisters, duration to the cessation of new blisters.
S: Hospital/clinic.
Inclusion and exclusion criteria
Studies were limited to randomized controlled trials comparing the efficacy of biologic agents to placebo or corticosteroids to treat oral lesions associated with pemphigus vulgaris/foliaceus, mucous membrane pemphigoid, Behcet's disease, oral lichen planus, or recurrent aphthous ulcers. Articles not available in English were excluded.
Search methods for identification of studies
Four electronic databases (MEDLINE via PubMed, EMBASE, Web of Science, and Cochrane Library) were searched on 2/27/2018 using the strategies described in Table 1. We re-ran the search for the four databases on 1/20/2019, and no relevant results were found.
Data collection and analysis
Three review authors (G.E.D., G.S., A.T.V.) screened titles and abstracts of the search results for inclusion/exclusion with a fourth author (R.E), making the final decision in case of disagreement. The same three review authors also independently extracted the data from the full-text articles meeting the inclusion criteria. Data extraction included the number of participants and their demographics, inclusion/exclusion criteria, interventions, and outcome data. The assessment of the risk of bias in the included studies was undertaken independently by the three authors (G.E.D., G.S., A.T.V.) in accordance with the approach described in the Cochrane Handbook21. The three assessments are low risk, unclear risk, and high risk for each of the following: 1) random sequence generation; 2) allocation concealment; 3) blinding; 4) incomplete outcome; 5) selective reporting; 6) other potential bias.
Statistical analyses
Meta-analyses could not be conducted due to heterogeneity of the conditions - pemphigus and Behcet's; the biologic interventions - etanercept22,23, infliximab24 and rituximab16; comparison groups - placebo injection22,23 or either prednisone alone16 or prednisone and placebo24; study design - double-blinded RCT22-24 or open-label RCT16 and outcomes measured.
Levels of evidence and summary of the review findings
Quality of evidence assessment and summary of the review findings were conducted following the Cochrane Collaboration and GRADE Working Group recommendations21. The GRADE Working Group grades of evidence are:
• High quality: Further research is very unlikely to change our confidence in the estimate of effect
• Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
• Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
• Very low quality: We are very uncertain about the estimate.
Results
Results of the search
The initial search strategy through database searching yielded 721 references plus 19 additional records identified through other sources (scanning of the reference section of included studies). 740 records were assessed independently by three review authors (G.E.D., G.D., A.T.V), and based on the abstracts and titles, these were reduced to 27 relevant manuscripts. All 27 manuscripts identified were analyzed for inclusion independently by the same three review authors. Four manuscripts were assessed as relevant for inclusion. The main reasons for exclusion are presented in the PRISMA flowchart (Figure 1). Table 1 provides a summary of the search strategy.
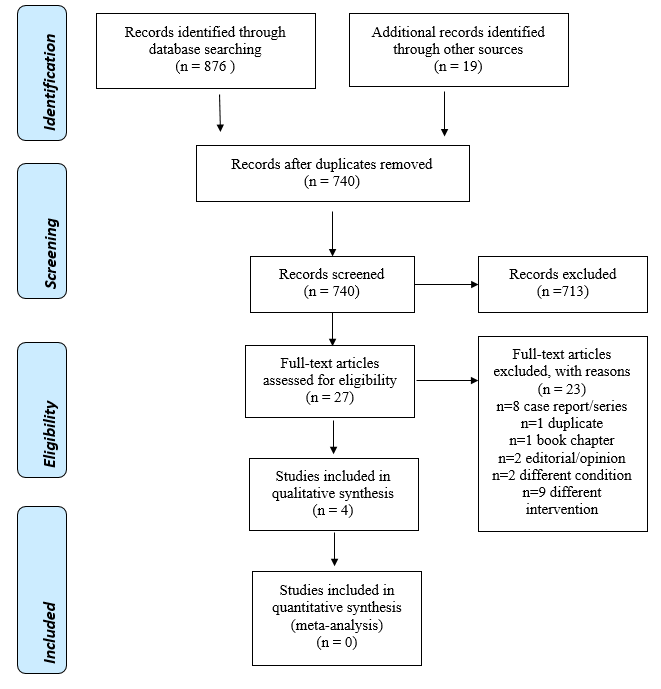
Figure 1: PRISMA flow diagram showing the number of abstracts identified, screened, eligible, and included.
Table 1: Search strategies
| Database | Search strategy |
|---|---|
| MEDLINE via PubMed (searched on 2/27/2018 and 1/20/2019) limited to English language and Humans | ("Biological Products"(Mesh) OR biological agent* OR monoclonal antibod* OR rituximab OR infliximab OR tocilizumab OR infliximab OR adalimumab OR etanercept OR golimumab OR certolizumab pegol OR tocilizumab OR abatacept OR daclizumab OR anakinra) AND (pemphigus OR mucous membrane pemphigoid OR oral lichen planus OR Behçet* OR recurrent aphthous ulcer* OR aphthous stomatitis) |
| The Web of Science (searched on 2/27/2018 and 1/20/2019) | (biological agent* OR monoclonal antibod* OR rituximab OR infliximab OR tocilizumab OR infliximab OR adalimumab OR etanercept OR golimumab OR certolizumab pegol OR tocilizumab OR abatacept OR daclizumab OR anakinra) AND (pemphigus OR mucous membrane pemphigoid OR oral lichen planus OR Behcet* OR recurrent aphthous ulcer* OR aphthous stomatitis) AND random* |
| The Cochrane Library (searched on 2/27/2018 and 1/20/2019) | #1. ((biological agent*) OR (monoclonal antibod*) OR rituximab OR infliximab OR tocilizumab OR infliximab OR adalimumab OR etanercept OR golimumab OR (certolizumab pegol) OR tocilizumab OR abatacept OR daclizumab OR anakinra) #2. (pemphigus OR (mucous membrane pemphigoid) OR (oral lichen planus) OR Behçet* OR (recurrent aphthous ulcer*) OR (aphthous stomatitis)) #3. Randomization OR randomized OR random #4. #1 and #2 and #3 |
| EMBASE (searched on 2/27/2018 and 1/20/2019) | #1.((biological agent*) OR (monoclonal antibod*) OR rituximab OR infliximab OR tocilizumab OR infliximab OR adalimumab OR etanercept OR golimumab OR (certolizumab pegol) OR tocilizumab OR abatacept OR daclizumab OR anakinra) #2. (pemphigus OR (mucous membrane pemphigoid) OR (oral lichen planus) OR Behçet* OR (recurrent aphthous ulcer*) OR (aphthous stomatitis)) #3. random* #4. #1 and #2 and #3 combine |
Included Studies
Although our original search included oral lesions associated with pemphigus vulgaris/foliaceus, MMP, Behcet's disease, oral lichen planus, or recurrent aphthous ulcers, only three RCTs on pemphigus vulgaris/foliaceus16,22,24 and one on Behcet's disease23 met inclusion criteria.
Study Design: A single open-label prospective multi-center parallel-group RCT16 and three double-blinded RCTs22-24 were eligible for qualitative analysis (Table 2).
Population: A combined total of 158 adults were enrolled in these four RCTs (138 pemphigus and 20 Behcet's disease patients). Patients’ diagnosis of pemphigus vulgaris/foliaceus was confirmed via direct immunofluorescence and histology16,22 or via disease activity score24. Behcet's disease was confirmed via monosodium urate (MSU) testing and pathergy23. All four RCTs included only adults; three of the RCTs enrolled both males and females16,22,24, while one, by design, included only male participants23. The average age of the participants ranged from 28.5 years23 to 54.5 years24. The inclusion criteria, as well as the average age and gender distribution of the included studies, are presented in Table 2.
Table 2: Summary of included RCT studies
| Reference | Country, Study Type | Intervention groups’ gender | Control groups’s gender | Inclusion criteria | Age, mean ± SD (range) |
|---|---|---|---|---|---|
| Pemphigus Vulgaris & Pemphigus Foliaceus | |||||
| Fiorentino et al. (2011) | United States, Pilot Study, DBRCT | 50mg Etanercept 5M/1F | Saline 0M/2F | Diagnosis: Pemphigus Vulgaris Age: >18; Location(s): Skin Biopsy Type: Skin Confirmation: Direct Immunofluorescence & Histology |
Etanercept: 54.17±10.26 Saline: 46.5 ± 11.5 |
| Hall et al. (2015) | United States, DBRCT | Infliximab (5mg/kg) + Prednisone (dosage varied) 6M/4F | Prednisone + placebo 6M/4F | Diagnosis: Pemphigus Vulgaris Age: >18; Location(s): Skin & Mucosal Confirmation: Disease activity score, mucosal and cutaneous, >/= 2 Timeframe: 2 Weeks prior to infusion |
Infliximab: 52 years (21-63) Prednisone: 54.5 years (30-71) |
| Joly et al. (2017) | France, Open-label RCT | 500-1,000mg IV Rituximab & 0.5-1.0 mg/kg daily prednisone 15M/ 31F | 1.0-1.5 mg/kg daily Prednisone 25M/19F | Diagnosis: Pemphigus Vulgaris & Pemphigus Foliaceus Age: 18-80; Location(s): N/A Confirmation: Direct Immunofluorescence & Histology Timeframe: New |
Rituximab: 53.5 years Prednisone: 53.1 years |
| Behcet's Disease | |||||
| Melikoglu et al. (2005) | Turkey, DBRCT | 25mg of Etanercept subcutaneous injection 20M/0F | Placebo 20M/0F | Diagnosis: Behcet's Disease Age: 18-45; Gender: Male Location(s): Skin (Genital) & Mucosal (Oral) Confirmation: Monosodium Urate (MSU) Test & Pathergy Timeframe: Last 3 Months |
Etanercept: 28.5±5.3 Placebo: 30.8±6.2 |
DBRCT=Double-blinded randomized controlled trial; M=Male; F=Female.
Interventions: Two RCTs randomized participants to receive etanercept22,23, one utilized infliximab24, and another utilized rituximab16 as their biologic intervention. In two trials16,24, the intervention included a corticosteroid, prednisone, along with the biologic medication. In one study16, prednisone dosing was based on disease severity (moderate versus severe), while the other study24 allowed the investigator to adjust the dose using the best medical judgment. Three RCTs utilized fixed doses of their respective intervention22-24, while one16 utilized a larger loading dose over two weeks followed by maintenance doses at 12 and 18 months.
Comparison group: Patients in the comparison groups were labeled as controls and received placebo injections22,23 or prednisone16 or prednisone plus placebo24.
Primary outcomes: Melikoglu et al.23 reported a primary endpoint of the amount of suppression of the pathergy response (reported as the number of positive responses out of total tested) and MSU test (reported as the area of erythema in mm2). In Hall et al. (2015), the primary efficacy endpoint was a response to treatment at week 18. Joly et al.16 reported the proportion of patients who achieved a complete remission at month 24. Finally, Fiorentino et al.22 reported the meantime to achieve a 50% reduction in the number of active lesions as the primary endpoint.
Risk of bias, outcomes and adverse events
Overall, all four included studies were considered at a high risk of bias. (Table 3). Table 4 presents the outcomes of the four included RCTs. Table 5 presents the adverse events reported in the four RCTs.
Table 3: Summary of risk of bias for included RCT studies.
| Study | Random Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | Other potential bias | Overall Bias |
|---|---|---|---|---|---|---|---|
| Fiorentino et al. (2011) | + | ? | + | + | + | + | + |
| Hall et al. (2015) | - | - | ? | + | - | - | + |
| Joly et al. (2017) | - | ? | + | + | - | ? | + |
| Melikoglu et al. (2005) | - | - | + | - | - | + | + |
Legend: - low risk; + high risk; ? unclear risk
Table 4: Primary outcomes reported in included RCT studies
| Reference | Interventions and Sample Size | Results |
|---|---|---|
| Pemphigus Vulgaris & Pemphigus Foliaceus | ||
| Fiorentino et al. (2011) | Group 1: IV 50mg etanercept (n=6) Group 2: Saline (n=2) |
• The pilot study was halted prematurely after enrollment of 8 participants. • 50% reduction in number of lesions was met by 3 of the 6 subjects completing the study (2 in the placebo group, 1 in the etanercept group). • Total number of lesions decreased more in the placebo group. • DLQI and PGA scores decreased for the treatment and the placebo groups. |
| Hall et al. (2015) | Group 1: IV Prednisone variable dosage + Infliximab (5mg/kg) (n=10) Group 2: Prednisone + placebo (n=10) |
• None of the primary or secondary efficacy endpoints reached statistical significance. • The primary efficacy endpoint at 18 weeks was achieved by only one subject in each group. • Three subjects in the treatment group did achieve responder status at week 26 compared with no subjects in the placebo group (P > 0.05). • Subjects treated with infliximab exhibited a decrease in the number of new blisters per week during the study whereas subjects treated with placebo showed an increase in the number of new blisters per • week (P = 0.037). • The treatment group achieved better scores in time to cessation of new blisters and healing of existing blisters, and less days to achieve event. • PDAI score improved more for the treatment group (week 18 P=.032, week 22 p=.008, week 26 p=.032) • IgG anti-Dsg1 and IgG anti-Dsg3 antibodies decreased significantly for the treatment group at week 18 (P = 0.035); week 26 (P = 0.022) • No significant differences in the DLQI scores • Less prednisone was needed to achieve cessation of new blister formation or healing of existing ulcerations/erosions in the treatment group |
| Joly et al. (2017) | Group 1: 500-1,000mg IV Rituximab plus short-term 0.5-1.0 mg/kg daily prednisone (n=46) Group 2: 1.0-1.5 mg/kg daily prednisone alone (n= 44) |
• More primary endpoint achievers in the treatment group (P<.0001) • Complete remission off-therapy achieved at 24 months close to 3 times more in the treatment group (89% vs. 28%, P<.0001). • Median delay to complete remission off-therapy and median duration of complete remission off-therapy were more than ½ lower and 7x higher respectively in the treatment group than the prednisone alone group (P<.0001). • Two-years disease-free survival was twice as high for the rituximab group (P=0.0191). • Cumulative dose for prednisone needed was close to 1/3 lower in the active group (p<.0001). • Total and desmoglein-specific B-lymphocytes disappeared in rituximab plus short-term prednisone group of patients, and remained unchanged in the prednisone-alone group. • Anti-desmoglein antibodies decreased for the active group and increased after the 12th month for the controls. • Prednisone cumulative dose post treatment was 3x higher in the control group (P<.0001). • At month 24, more relapse cases were recorded in the control group. |
| Behcet's Disease | ||
| Melikoglu et al. (2005) | Group 1: 25mg of etanercept subcutaneous injection (n=20) Group 2: Placebo (n=20) |
• The frequency of pathergy was not different between the two groups at any time. • The area of erythema by MSU was less in the placebo group at Week 1 and no different than the etanercept group at Week 4 and at the third month after the end of the study. MSU was lower for both study arms at the end of the third month. • The numbers of oral ulcers were less in the treatment group at weeks 1, 2, 3 and 4. Post-study, both groups returned to pretreatment levels. • 45% (9/20) of etanercept patients were free of oral ulcers and only 5% (1/20) of the control group (p=0.0017). |
Abbreviations:
DLQI: dermatology life quality index
PGA: Physician global assessment (scale: 0, indicates clear;1, near clear; 2, mild disease; 3, moderate disease; and 4, severe disease)
PDAI: Pemphigus Disease Area Index
RR: relative risk
NNT: Number Needed to Treat
Skindex measures the effects of skin diseases on patients’ quality of life.
Pathergy positivity: a papule or pustule formation 48 h after the insertion of a 20 gauge needle
MSU positivity: an area of erythema that formed after subcutaneous injection of 2.5 mg MSU crystals.
Table 5: Adverse events (AEs)
| Reference | Interventions and Sample Size | Adverse Events in the Intervention Group | Adverse Events in the Placebo group |
|---|---|---|---|
| Pemphigus Vulgaris & Pemphigus Foliaceus | |||
| Fiorentino et al. (2011) | Group 1: IV 50mg etanercept (n=6) Group 2: Saline (n=2) |
1 hip fracture 1 inj. site reaction 1 ecchymosis and numbness at injection site 1 pemphigus vulgaris flare at week two 1 superficial skin infection |
1 headache 2 cases of inj. site reaction |
| Hall et al. (2015) | Group 1: IV prednisone variable dosage + Infliximab (5mg/kg) (n=10) Group 2: Prednisone + placebo (n=10) |
0 AEs > grade 3 on or before 18 weeks 1 Severe adverse event grade 3 |
2 AEs grade 3 1 AE grade 4 1 AE grade 5 |
| Joly et al. (2017) | Group 1: 500-1,000mg IV rituximab plus short-term 0.5-1.0 mg/kg daily prednisone (n=46) Group 2: 1.0-1.5 mg/kg daily prednisone alone (n= 44) |
(27 SEVERE events grade > 3 in 16 patients) 6 diabetes and endocrine disorder (22%) 3 myopathy (11%) 5 bone disorders (19%) |
(53 SEVERE events grade > 3 in 29 patients) 11 diabetes and endocrine disorder (21%) 10 myopathy (19%) 5 bone disorders (9%) |
| Behcet's Disease | |||
| Melikoglu et al. (2005) | Group 1: 25mg of etanercept inj. (n=10) Group 2: Placebo (n=10) |
1 diarrhea 1 gastrointestinal abdominal pain with bloody diarrhea with ileocecal ulcer on endoscopy |
1 patient had elevated liver enzymes (ALT 144 U/l, AST 71 U/l) and a positive hepatitis C virus alongside a negative HCV-RNA, 3 months after the study ended |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; inj: injection
Summary of the evidence and quality of the findings
The quality of evidence was very low due to the high risk of bias, heterogeneity of the outcomes (only one study reported each outcome of interest), and the small number of studies with a small sample size. Very low evidence grading indicates that we are very uncertain about the estimate of effect.
Discussion
Summary of the results
A single open-label prospective multi-center parallel-group RCT16 and three double-blinded RCTs22-24 were included in this review. A total of 158 subjects in the studies were treated for pemphigus vulgaris/foliaceus (138 patients)16,22,24 or Behcet's (20 patients)23.
Pemphigus vulgaris/foliaceus: Patients were treated either by IV infliximab plus prednisone24, IV rituximab plus prednisone16, or subcutaneous etanercept22. The results were inconclusive when etanercept alone was the active treatment22. When infliximab was added to prednisone, a statistically significant improvement was recorded with the Pemphigus Disease Area Index, the Dermatology Quality of Life Index, and Skindex scores; however, this was not the primary endpoint and was evaluated on a per-protocol basis24. A complete remission at 24 months, the median duration of complete remission, and two years of disease-free survival were all higher with IV rituximab plus prednisone compared to prednisone alone control group16. Less prednisone was needed to achieve the desired outcomes with the addition of rituximab16 or infliximab24 to prednisone. IgG anti-Dsg1 and Dsg3 antibodies24 or total and desmoglein-specific B-lymphocytes16 were reduced or disappeared in infliximab plus prednisone or rituximab plus prednisone groups of patients compared to prednisone alone. Based on these results, IV rituximab plus prednisone or IV infliximab plus prednisone may be a superior treatment compared to prednisone alone.
Behcet's Disease: Etanercept was more effective in reducing the number of oral ulcers and achieving complete remission than placebo23.
Adverse effects
Infliximab with prednisone24 and rituximab with short-term prednisone16 showed higher safety than prednisone alone in the treatment of pemphigus. The higher safety is likely delivered by the lower dose of prednisone when infliximab or rituximab is added to the treatment. More adverse events were reported when etanercept was utilized for the treatment for pemphigus vs. placebo controls22. The number of severe complications was lower, and the overall complications were higher in the etanercept group compared to the placebo in the treatment of Behcet's disease. The active treatment group reported abdominal pain, bloody diarrhea, and an ileocecal ulcer23.
Agreements and disagreements with other studies or reviews
Only RCTs were included in this systematic review. Five clinical trials were excluded from this review for lack of control group, two were retrospective studies25,26, and three were uncontrolled prospective studies27-29. Three clinical trials found rituximab to be beneficial in the treatment of recalcitrant pemphigus25,26,28 with early treatment within six months of diagnosis being more beneficial26. One of the uncontrolled studies with 49 MMP patients (24 receiving rituximab as adjuvant therapy and 25 using conventional therapy), concluded that the addition of rituximab to conventional therapy resulted in faster and more sustained disease control with potentially fewer adverse events29. An open-label pilot study with four patients with erosive lichen planus treated via subcutaneous efalizumab showed that two patients experienced serious adverse events27. One patient experienced urticaria and a staphylococcal abscess of an artificial hip joint. The second patient had drug-induced subacute cutaneous lupus27.
The findings of this systematic review are in agreement with other reviews; that biologic agents offer either an alternative treatment to or adjuvant treatment with glucocorticoids; however, not without risks and significant adverse effects30,31. A recent systematic review also identified favorable results when administering rituximab for treating pemphigus32. Two reviews concluded that rituximab and a short course of corticosteroids are more effective and safer than standard corticosteroids treatment, leading to its approval as first-line therapy in pemphigus by the FDA in 201833,34. According to one review35, and a systematic review36, MMP is less responsive to rituximab than pemphigus. Further studies are needed.
Only one RCT with etanercept has shown beneficial results for Behcet's disease (included in this review23) with few adverse events37. Infliximab and adalimumab are increasingly used for various refractory Behcet's syndrome manifestations despite the lack of controlled studies, according to one review38.
Analysis of the influence of risk of bias on the results: Quality of the Evidence
The overall quality of the evidence (according to the GRADE system) was very low due to the risk of bias (which was high in all studies), a small number of randomized controlled trials for each outcome (n=1), a small sample size (less than 400 total number of participants), heterogeneity of the outcomes reported and quality of study designs. While three DBRCT were included in this review22-24, one included study16 was an open-label randomized trial. Additionally, one trial was a pilot study22. Further randomized controlled trials of a larger scale are needed to improve the quality of the data.
Implications for research and clinical practice
Infliximab plus prednisone and rituximab plus prednisone were more effective and safer compared to prednisone alone for the treatment of moderate to severe pemphigus. The reduced prednisone dose may be the contributing factor for the lower side effects achieved with the addition of the biologic agents. Those results make these treatments a viable alternative for refractory cases of pemphigus or cases where higher doses of prednisone are contraindicated. In June 2018, the US Food and Drug Administration approved rituximab for the treatment of adults with moderate to severe pemphigus vulgaris. Both systemic steroids and rituximab are considered first-line treatment39. According to the European Dermatology Forum, rituximab is indicated in patients who remain dependent on more than 10 mg prednisolone combined with an immunosuppressive adjuvant40. Using biologics, in general, allows for more rapid tapering of systemic steroid doses and a major steroid-sparing39. Research related to maintenance infusions of rituximab or infliximab is indicated as infusion may keep B-lymphocytes and IgG anti-Dsg1 and Dsg3 antibodies at the desired levels and reducing relapse. Similarly, etanercept is showing a reduction of relapses in refractory Behcet's disease41.
Infliximab binds to soluble and transmembrane forms of TNF-α, which prevents TNF-α from interacting with its receptors. The therapeutic role of infliximab is an anti-inflammatory role by downregulation of local and systemic pro-inflammatory cytokines (i.e., IL-1, IL-6), and reduction of lymphocyte and leukocyte migration to sites of inflammation17. On the other hand, desmoglein antigens, which play an essential role in the pathogenesis of pemphigus, are produced by plasma cells developed from autoreactive B-cells; Rituximab may act by binding to the CD20 cell surface receptor of B-cells and destroy them18. Thus the therapeutic role of rituximab is suppressing the autoimmune reaction against the skin or/and mucosa. The long term safety, cost, and efficacy of the infusions need to be further investigated.
Etanercept, which is a receptor fusion protein that inhibits TNF-α, was an effective treatment in reducing the number of oral ulcers and achieving complete remission in Behcet's disease in one study compared to placebo23. However, the effect of etanercept on women with Behcet's needs to be investigated, as the study did not include women. Etanercept was more effective in the treatment of oral ulcers than ulcers in other body locations in patients with Behcet's disease. Although the effectiveness of etanercept for pemphigus patients is heterogeneous, it has a favorable outcome with Behcet's patients19. Why etanercept was effective for Behcet's while ineffective for pemphigus may be due to the difference in pathogenesis between these conditions and/or inadequate dosing/administration.
Further research in optimal dosing, selection, and administration of biologic treatments is necessary. This is a relatively new therapy with an unknown cost. Finally, well designed and high-quality studies limited to oral manifestations of these conditions will allow for improved clinical application and usefulness.
In conclusion, this systematic review found the use of biologic agents as an adjunct to prednisone may reduce the amount of required steroid, thus reducing the adverse effects of steroid usage in pemphigus patients. However, more studies are needed to confirm the benefits of biologic agents, considering the heterogeneity of the included research (conditions, interventions, outcomes, comparison groups, and adverse events). Although the addition of biologic agents to prednisone may show promise, the current evidence is inadequate to support the routine administration of biologic medications as first-line therapy for the prevention or treatment of Behcet's disease.
Acknowledgments
The authors would like to thank Dr. James E. Cade, DDS, Meharry Medical College School of Dentistry for his helpful review of the manuscript. This systematic review was registered with PROSPERO #CRD42019128312.
Conflict of Interest
There was no financial support for this review, and the authors have no conflicts of interest to declare.
Abbreviations
RCT: Randomized controlled trial; DBRCT: Double-blinded Randomized Controlled Trial; MSU: monosodium urate; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
References
- Jimson S, Balachader N, Anita N, et al. Immunologically mediated oral diseases. J Pharm Bioall Sci. 2015; 7: S209-12. doi:10.4103/0975-7406.155909
- Kilic A. Pemphigus: Subtypes, Clinical Features, Diagnosis, and Treatment. Autoimmune Bullous Dis. 2018. doi:10.5772/intechopen.71712
- Santoro FA, Stoopler ET, Werth VP. Pemphigus. Dent Clin North Am. 2013; 57(4): 597-610. doi:10.1016/j.cden.2013.06.002
- Nousari HC, Anhalt GJ. Pemphigus and bullous pemphigoid. Lancet. 1999; 354(9179): 667-672. doi:10.1016/S0140-6736(99)03007-X
- Amagai M, Nishikawa T, Nousari HC, et al. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998; 102(4): 775-782. doi:10.1172/JCI3647
- Becet H. [Uber rezidivierende, aphthose, dürch ein Virus versachte Geshwure am Munde, am Auge und an den Genitalien]. German. Dermatologische Wochenschrift. 1937; 105: 1152-1163.
- Sakane T. New perspective on Behcet’s disease. Int Rev Immunol. 1997; 14(1): 89-96. doi:10.3109/08830189709116847
- Torchia D, Caproni M, Fabbri P. Linear IgA disease and desquamative gingivitis: Time for inclusion in mucous membrane pemphigoid. Oral Dis. 2008; 14(8): 768-769. doi:10.1111/j.1601-0825.2008.01485.x
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: A multicenter cohort analysis. Medicine (Baltimore). 2010; 89(3): 176-182. doi:10.1097/MD.0b013e3181dfca14
- Carbone M, Arduino PG, Carrozzo M, et al. Topical clobetasol in the treatment of atrophic-erosive oral lichen planus: A randomized controlled trial to compare two preparations with different concentrations. J Oral Pathol Med. 2009; 38(2): 227-233. doi:10.1111/j.1600-0714.2008.00688.x
- Chams-Davatchi C, Esmaili N, Daneshpazhooh M, et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J Am Acad Dermatol. 2007; 57(4): 622-628. doi:10.1016/j.jaad.2007.05.024
- Chan LS, Ahmed AR, Anhalt GJ, et al. The First International Consensus on Mucous Membrane Pemphigoid. Arch Dermatol. 2002; 138(3). doi:10.1001/archderm.138.3.370
- Fani MM, Ebrahimi H, Pourshahidi S, et al. Comparing the Effect of Phenytoin Syrup and Triamcinolone Acetonide Ointment on Aphthous Ulcers in Patients with Behcet’s Syndrome. Iran Red Crescent Med J. 2012; 14(2): 75-78.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002; 96(1): 23-43. doi:10.1016/S0163-7258(02)00297-8
- Almugairen N, Hospital V, Bedane C, et al. Assessment of the rate of long-term complete remission off therapy in patients with pemphigus treated with different regimens including medium- and high-dose corticosteroids. J Am Acad Dermatol. 2013; 69(4): 583-588. doi:10.1016/j.jaad.2013.05.016
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017; 389(10083): 2031-2040. doi:10.1016/S0140-6736(17)30070-3
- Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993; 30(16): 1443-1453. doi:10.1016/0161-5890(93)90106-L
- Perosa F, Favoino E, Caragnano MA, et al. CD20: A target antigen for immunotherapy of autoimmune diseases. Autoimmun Rev. 2005; 4(8): 526-531. doi:10.1016/j.autrev.2005.04.004
- Marotte H, Cimaz R. Etanercept – TNF receptor and IgG1 Fc fusion protein: is it different from other TNF blockers? Expert Opin Biol Ther. 2014; 14(5): 569-572. doi:10.1517/14712598.2014.896334
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7): e1000097. doi:10.1371/journal.pmed.1000097
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011].; 2011. www.cochrane-handbook.org.
- Fiorentino DF, Garcia MS, Rehmus W, et al. A Pilot Study of Etanercept Treatment for Pemphigus Vulgaris. Arch Dermatol. 2010; 147(1): 117. doi:10.1001/archdermatol.2010.409
- Melikoglu M, Fresko I, Mat C, et al. Short-term trial of etanercept in Behcet’s disease: A double blind, placebo controlled study. J Rheumatol. 2005; 32(1): 98-105. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L40092599.
- Hall RP 3rd, Fairley J, Woodley D, et al. A multicentre randomized trial of the treatment of patients with pemphigus vulgaris with infliximab and prednisone compared with prednisone alone. Br J Dermatol. 2015; 172(3): 760-768. doi:10.1111/bjd.13350
- Arduino PG, Broccoletti R, Carbone M, et al. Long-term evaluation of pemphigus vulgaris: A retrospective consideration of 98 patients treated in an oral medicine unit in north-west Italy. J Oral Pathol Med. 2019; 48(5): 406-412. doi:10.1111/jop.12847
- Balighi K, Daneshpazhooh M, Akbari Z, et al. Comparing the short-term therapeutic effects and safety profiles of rituximab therapy in pemphigus vulgaris patients either early treated or later than six months. J Dermatolog Treat. 2019; 30(4): 346-349. doi:10.1080/09546634.2018.1509049
- Heffernan MP, Smith DI, Bentley D, et al. A single-center, open-label, prospective pilot study of subcutaneous efalizumab for oral erosive lichen planus. J Drugs Dermatol. 2007; 6(3): 310-314.
- Kanwar AJ, Vinay K, Sawatkar GU, et al. Clinical and immunological outcomes of high- and low-dose rituximab treatments in patients with pemphigus: a randomized, comparative, observer-blinded study. Br J Dermatol. 2014; 170(6): 1341-1349. doi:10.1111/bjd.12972
- Maley A, Warren M, Haberman I, et al. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid (MMP). J Am Acad Dermatol. 2016; 74(5): 835-840. doi:10.1016/j.jaad.2016.01.020
- O’Neill ID. Off-label use of biologicals in the management of inflammatory oral mucosal disease. J oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2008; 37(10): 575-581. doi:10.1111/j.1600-0714.2008.00693.x
- O’Neill ID, Scully CIDOCS. Biologics in oral medicine: Ulcerative disorders. Oral Dis. 2013; 19(1): 37-45. doi:10.1111/j.1601-0825.2012.01931.x
- Kaegi C, Wuest B, Schreiner J, et al. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front Immunol. 2019; 10(September): 1-18. doi:10.3389/fimmu.2019.01990
- Bilgic A, Murrell DF. What is novel in the clinical management of pemphigus. Expert Rev Clin Pharmacol. 2019; 12(10): 973-980. doi:10.1080/17512433.2019.1670059
- Didona D, Maglie R, Eming R, et al. Pemphigus: Current and future therapeutic strategies. Front Immunol. 2019; 10(June): 1-28. doi:10.3389/fimmu.2019.01418
- Buonavoglia A, Leone P, Dammacco R, et al. Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmun Rev. 2019; 18(4): 349-358. doi:10.1016/j.autrev.2019.02.005
- Mays JW, Carey BP, Posey R, et al. World Workshop of Oral Medicine VII: A systematic review of immunobiologic therapy for oral manifestations of pemphigoid and pemphigus. Oral Dis. 2019; 25(S1): 111-121. doi:10.1111/odi.13083
- Leccese P, Ozguler Y, Christensen R, et al. Management of skin, mucosa and joint involvement of Behcet's syndrome: A systematic review for update of the EULAR recommendations for the management of Behcet,s syndrome. Semin Arthritis Rheum. 2019; 48(4): 752-762. doi:10.1016/j.semarthrit.2018.05.008
- Esatoglu SN, Hatemi G. Update on the treatment of Behcet's syndrome. Intern Emerg Med. 2019; 14(5): 661-675. doi:10.1007/s11739-019-02035-1
- Murrell DF, Peña S, Joly P, et al. Diagnosis and management of pemphigus: Recommendations of an international panel of experts. J Am Acad Dermatol. 2020; 82(3): 575-585.e1. doi:10.1016/j.jaad.2018.02.021
- Hertl M, Jedlickova H, Karpati S, et al. Pemphigus. S2 Guideline for diagnosis and treatment - Guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatology Venereol. 2015; 29(3): 405-414. doi:10.1111/jdv.12772
- Ma D, Zhang CJ, Wang RP, et al. Etanercept in the Treatment of Intestinal Behcet’s Disease. Cell Biochem Biophys. 2014; 69(3): 735-739. doi:10.1007/s12013-014-9860-4